
This makes it easier to understand and predict how atoms will interact to form chemical bonds. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This give us the (correct) configuration of:įor the Cr 2+ ion we remove one electron from 4s1 and one from the 3d5 leaving us with:įor the Cr 3+ ion we remove a total of three electrons (one from the 4s1 and two from the 3d5) leaving us with Therefore, one of the 4s2 electrons jumps to the 3d5 so that it is half-filled (see video below).
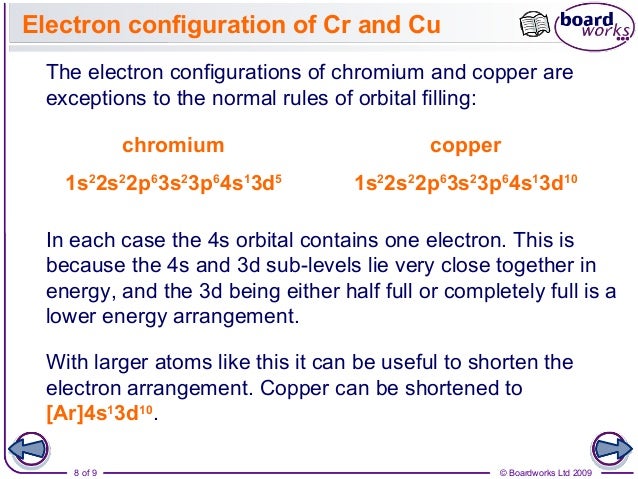
Half-filled and fully filled subshell have got extra stability. Therefore we have (still incorrect) 1s 22s 22p 63s 23p 63d 44s 2Ĭorrect Electron Configuration for Chromium (Cr) Both of the configurations have the correct numbers of electrons in each orbital, it is just a matter of how the electronic configuration notation is written ( here is an explanation why). Note that when writing the electron configuration for an atom like Cr, the 3d is usually written before the 4s. Therefore the expected electron configuration for Chromium will be 1s 22s 22p 63s 23p 44s 23d 9.
#Chromium valence electrons full#
After the 4s is full we put the remaining four electrons in the 3d orbital and end with 3d4. We now shift to the 4s orbital where we place the remaining two electrons. Since the 3s if now full we'll move to the 3p where we'll place the next six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. The p orbital can hold up to six electrons. The next six electrons will go in the 2p orbital.
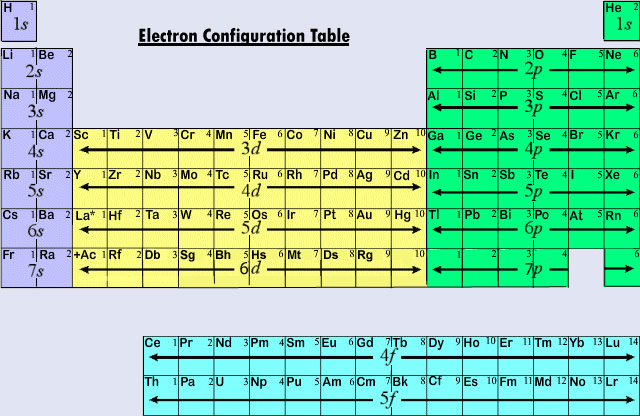
Below are their quantum numbers (N - energy, L - angular momentum, M - magnetic moment, S - spin ). Potassium has nineteen electrons, one more than the noble gas. The fourth and subsequent periods follow the same pattern, except for the use of a different noble gas. Again, the number of valence electrons increases from one to eight across the third period. Since 1s can only hold two electrons the next 2 electrons for Chromium go in the 2s orbital. The number of valence electrons in a Chromium atom - 6. 1 shows the noble gas configurations of the third period elements. In writing the electron configuration for Chromium the first two electrons will go in the 1s orbital. Step 2: Identify the electron of interest, and ignore all electrons in higher groups (to the right in the list from Step 1). Video: Cr, Cr 2+, and Cr 3+ Electron Configuration Notation Step 1: Write the electron configuration of the atom in the following form: (1s) (2s, 2p) (3s, 3p) (3d) (4s, 4p) (4d) (4f) (5s, 5p).


 0 kommentar(er)
0 kommentar(er)
